Votre e-mail a été envoyé.
Certaines informations ont été traduites automatiquement.
INFORMATIONS PRINCIPALES
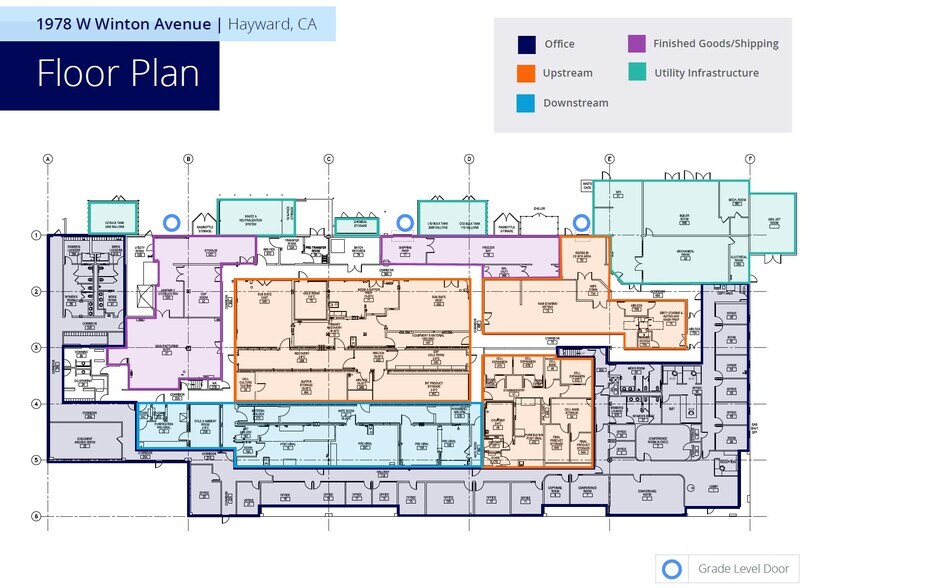
- Power and utilities to operate 2 x 1,000L and 2 x 2,000L single-use bioreactors, enabling flexible, high-yield production.
CARACTÉRISTIQUES
TOUS LES ESPACE DISPONIBLES(1)
Afficher les loyers en
- ESPACE
- SURFACE
- DURÉE
- LOYER
- TYPE DE BIEN
- ÉTAT
- DISPONIBLE
Previously operated by Lonza, this turnkey multiproduct cGMP facility is purpose-built for clinical-stage biopharmaceutical manufacturing specializing in the production of biotherapeutics, including antibodies, fusion proteins, and enzymes, using mammalian cell culture. The facility supports technology transfer, single-use manufacturing, and comprehen-sive analytical and regulatory services. Power (1,000 amps @ 277/480v) Backup Generator (1,000 gallons) Connectivity for WFI system previously outfitted with 10kL HWFI storage/recirculation tank and previously installed HWFI loop and POU heat exchangers to ambient WFI. New Chiller supporting cold rooms 3rd party bulk gas service (O2, CO2 and LN2) Upgraded Boilers
- Le loyer ne comprend pas les services publics, les frais immobiliers ou les services de l’immeuble.
- Lift pump for waste to central neutralization
- Toilettes privées
| Espace | Surface | Durée | Loyer | Type de bien | État | Disponible |
| 1er étage | 3 592 m² | Négociable | 331,94 € /m²/an 27,66 € /m²/mois 1 192 319 € /an 99 360 € /mois | Local d’activités | Construction achevée | Maintenant |
1er étage
| Surface |
| 3 592 m² |
| Durée |
| Négociable |
| Loyer |
| 331,94 € /m²/an 27,66 € /m²/mois 1 192 319 € /an 99 360 € /mois |
| Type de bien |
| Local d’activités |
| État |
| Construction achevée |
| Disponible |
| Maintenant |
1er étage
| Surface | 3 592 m² |
| Durée | Négociable |
| Loyer | 331,94 € /m²/an |
| Type de bien | Local d’activités |
| État | Construction achevée |
| Disponible | Maintenant |
Previously operated by Lonza, this turnkey multiproduct cGMP facility is purpose-built for clinical-stage biopharmaceutical manufacturing specializing in the production of biotherapeutics, including antibodies, fusion proteins, and enzymes, using mammalian cell culture. The facility supports technology transfer, single-use manufacturing, and comprehen-sive analytical and regulatory services. Power (1,000 amps @ 277/480v) Backup Generator (1,000 gallons) Connectivity for WFI system previously outfitted with 10kL HWFI storage/recirculation tank and previously installed HWFI loop and POU heat exchangers to ambient WFI. New Chiller supporting cold rooms 3rd party bulk gas service (O2, CO2 and LN2) Upgraded Boilers
- Le loyer ne comprend pas les services publics, les frais immobiliers ou les services de l’immeuble.
- Toilettes privées
- Lift pump for waste to central neutralization
APERÇU DU BIEN
Previously operated by Lonza, this turnkey multiproduct cGMP facility is purpose-built for clinical-stage biopharmaceutical manufacturing specializing in the production of biotherapeutics, including antibodies, fusion proteins, and enzymes, using mammalian cell culture. The facility supports technology transfer, single-use manufacturing, and comprehensive analytical and regulatory services. • Upstream Processing: power and utili-ties to operate 2 x 1,000L and 2 x 2,000L single-use bioreactors, enabling flexible, high-yield production. • Downstream Processing: Robust purification capabilities to ensure product quality and compliance. • Support Services: Includes pilot labs, analytical testing, stability studies, quality control, and regulatory support for seamless process development. • Aseptic Filling: Single-use technology for bulk drug substance filling into bags and bottles, with frozen or refrigerated storage options. • Infrastructure: Designed for 20/7 operations, offering scalability for future teams. • Location Advantage: Strategically located in Hayward, CA, with proximity to Bay Area Life Sciences hubs Existing Infrastructure: • Power (1,000 amps @ 277/480v) • Backup Generator (1,000 gallons) • Connectivity for WFI system previously outfitted with 10kL HWFI storage/recir-culation tank and previously installed HWFI loop and POU heat exchangers to ambient WFI. • New Chiller supporting cold rooms • 3rd party bulk gas service (O2, CO2 and LN2) • Upgraded Boilers • Lift pump for waste to central neutralization system
INFORMATIONS SUR L’IMMEUBLE
Présenté par

1978 W Winton Ave
Hum, une erreur s’est produite lors de l’envoi de votre message. Veuillez réessayer.
Merci ! Votre message a été envoyé.